Abstract
Keywords: Sheep; Trans Humane; Inconsistent Abortion; Brucellosis; Blue Tongue; Confounding
Introduction
Materials and Methods
Study Area and Flock Details
This study was carried out during the period between November 2015 to November 2016 in the Cavery delta districts of Tamil nadu, India. These delta districts are the rice bowl of Tamil nadu state and accounts for 75% of the state’s rice production. The rice harvesting season attracts the several Sheep shepherds to this area to graze the paddy fields after harvesting. Trans humane sheep flocks having flock strength ranging from 300 to 5000 animals per ownership are common sights in these areas. Each flock may have about 300 sheep 25 goat and 9 cattle (Figure 1). These animals were not immunized against brucellosis and blue tongue and no periodical deworming programs are followed. In fact no organized animal health care is being practiced by such nomadic farmers.Choice of Samples
Samples were collected from pregnant non aborted, pregnant aborted, non pregnant ewes and rams. For sample size calculation, expected prevalence of brucellosis was assumed as 20 percent with five percent absolute frequency.Sampling Methods
Clinical materials like whole blood in EDTA tubes, serum collected in clot activator tubes aseptically and stored in refrigeration until further processing. Simple random sampling method was used for sampling.Brucellosis Screening
Clinical samples like serum samples were collected and submitted for Brucellosis, Blue tongue, Chalymidiasis, Malignant catarrahal fever and Bovine viral diarrhoea diseases since the season and clinical history had given clues for such pathogens. Rose Bengal plate agglutination test (RBT) and I ELISA tests undertaken for brucellosis identification.Blue Tongue Screening
For blue tongue identification, whole blood in EDTA was collected and subjected to polymerase chain reaction.Bovine Viral Diarrhoea (BVD) Malignant Cattarhal Fever (MCF) and Chalymidiasis Screening
Serum samples were collected aseptically and analyzed by nested PCR for BVD and MCF. Serology was carried out for Chalymidiasis.Test Protocols
Rose Bengal plate agglutination (RBT) was carried out as per the OIE prescribed procedure. The recommended steps to improve sensitivity of RBT by using three volumes of serum and one volume of antigen (e.g. 75μl and 25μl, respectively) in place of an equal volume of each (Figure 2) This was used in this study modification helped in increasing RBT sensitivity and minimized the discrepancies between RBT and other diagnostic tests. Positive serum and antigen purchased from Indian Veterinary Research Institute (IVRI), Bareilly, India was used in this study. Monoclonal based blocking ELISA for diagnosis of brucellosis was adopted as per the manufactures protocol. For Blue tongue Polymerase chain reaction was adopted as per the standard procedure of OIE.Results
Discussion
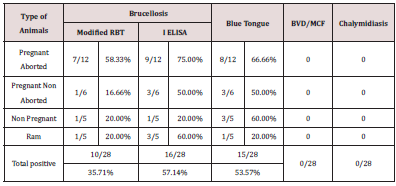
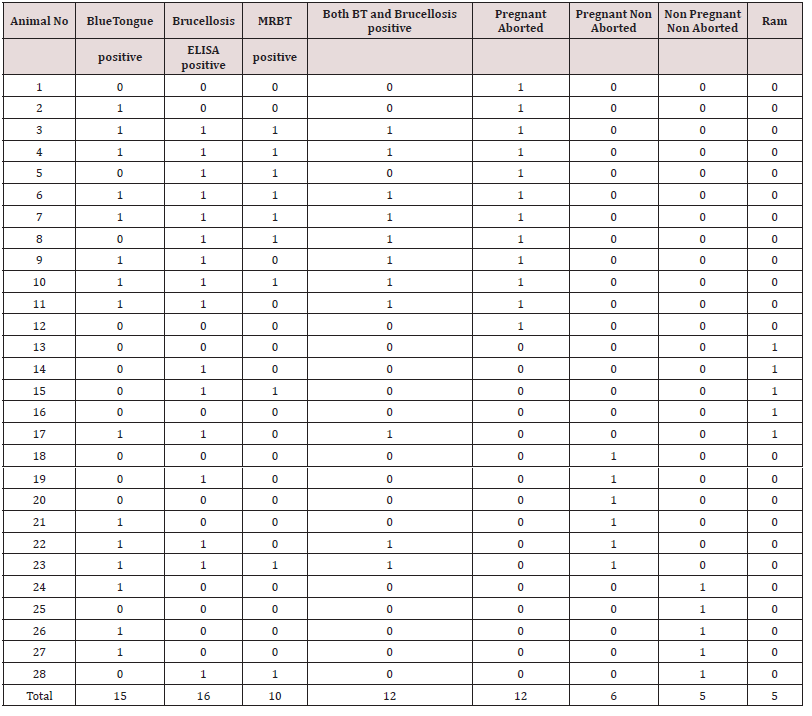
In India mostly the sero positive animals were handled based on “test and separate” policy rather than the “test and slaughter” policy due to economic concerns of these marginalized nomadic people. Unplanned immunization program coupled with no effective quarantine and uncontrolled trans-state migration of animals are major factors that affects the Brucellosis control programs [9]. Host susceptibility is also variable with the reproductive status. Thus in the field level, all intermediate stages between typical acute infection to complete resistance may be observed. Under pregnant aborted animals category 58.3% and 66.66% animals showed positivity for Brucellosis and Bluetongue respectively and this highlighted that there is certain confounding effect which makes the animal to abort. In pregnant non aborted category lower percentage of animals showed positive for both Brucellosis and Bluetongue which further supports the possibilities of confounding phenomenon augmenting abortion feature. Interesting by aborted animals got pregnant in their next season and gave birth to normal lambs possibly many animals may develop self limiting infections or they become asymptomatic latent carriers and turn in to potential source of future infections. Abortion generally does not occur if the female is infected at the last stage of pregnancy. Non pregnant non aborted animals showed higher Blue tongue positivity than brucellosis which enlightenend the real risk factor for abortion. Non-pregnant animals exposed to small numbers of organisms may develop selflimiting, immunizing infections or they may become latent carriers [12,13]. No proper selection of breeding ram between flocks of Sheep, low /no biosecurity measures, poor awareness about the vaccination had resulted in brucellosis becoming a continuous threat to the trans humane Sheep populations. As 60 % of rams, showed seropositivity for brucellosis, they posed a great threat to the breeding ewes. Interestingly non of the rams showed clinical signs of Brucellosis. Asymptomatically infected rams deserve to have poor fertility and contributes to dissemination of B. ovis and when very high percentage of rams were infected marked infertility becomes evident in the flock [14]. Seroconversion and shedding of bacteria in the semen of infected rams demonstrated mild or no detectable lesions during the acute phase of infection [15].
The study period of October month had a higher culicids’ populations and it in turn would have helped in transmission of Blue tongue. Results here indicated that BTV is epidemiologically important, and further studies are required to determine the true spatial distribution and cause for abortion in Sheep. Unplanned and uncontrolled grazing and frequent addition of flocks of sheep also contribute to the wide distribution of brucellosis in these animals. Even though the goat and cattle reared along with sheep flock, they were not part of this study, however they may had also acted as a cofounder for Brucellosis. Future studies on these aspects will add more information.
To know more about our Veterinary Sciences click
on https://lupinepublishers.com/dairy-veterinary-science-journal